NAVIGATE ESUS
Primary research question: Is rivaroxaban superior to ASA for the prevention of recurrent strokes in patients following an initial embolic stroke of unknown source (ESUS)?
Study design: Double-blinded randomized control trial
Population
- Number of patients studied: 7213
- Inclusion criteria: Aged ≥50 years; If <60 years, ≥ 1 vascular risk factor, including hypertension, DM, prior stroke, smoking, or HF
- Exclusion criteria: As above, the index stroke: Wasn't lacunar and wasn't associated; Wasn't associated with >50% stenosis in extracranial vessel supplying the affected region; No obvious cardiac source; Severely disabling stroke; Indication for chronic anticoagulation or antiplatelet therapy; eGFR <30 mL/min/1.73 m2; Ongoing regular use of NSAIDs; Major bleeding within the previous 6 months; Previous nontraumatic intracranial hemorrhage; Current or planned implantable loop recorder placement; Coagulopathy from liver disease
Interventions
- Experimental group: Rivaroxaban - 15 mg po qday
- Control group: Aspirin - 100 mg daily
Primary outcome
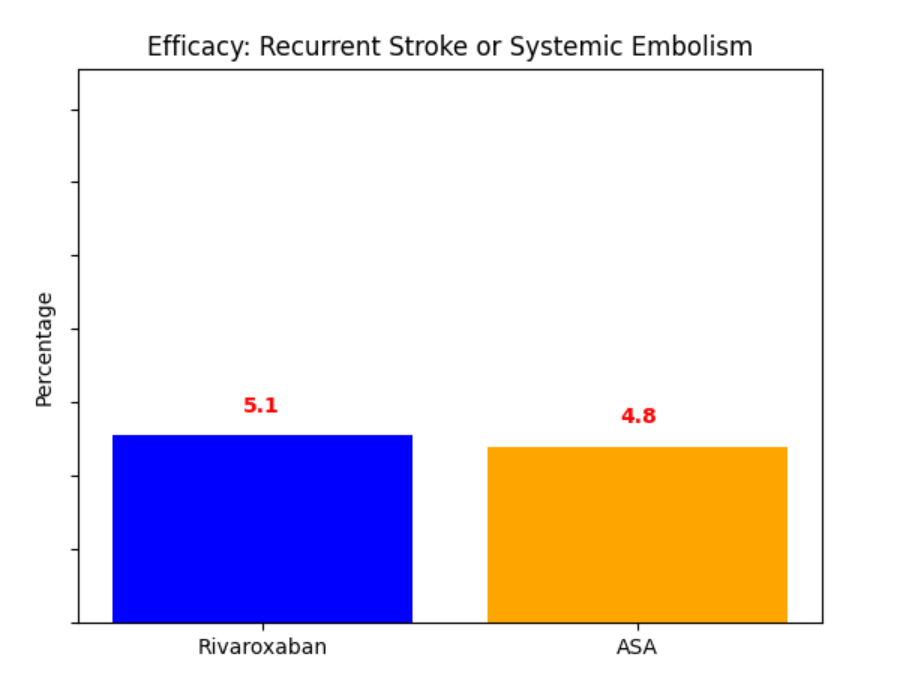
No significant difference in the primary efficacy outcome. However, there was a significant increase in major bleeding in the rivaroxaban group.
Secondary outcomes
Stroke type, systemic embolism, MI, any stroke, MI, CVD mortality, or systemic embolism, and mortality.
Safety outcomes or Adverse Events
Major bleeding, clinically relevant non-major bleeding, and any bleeding were the safety outcomes.
Conclusion
In patients with embolic stroke of unknown source, rivaroxaban was not superior to aspirin in preventing recurrent stroke. However, rivaroxaban was associated with a higher risk of major bleeding compared to aspirin.
Hart, R. G., Sharma, M., Mundl, H., Kasner, S. E., Bangdiwala, S. I., Berkowitz, S. D., ... & Lavados, P. M. (2018). Rivaroxaban for stroke prevention after embolic stroke of undetermined source. New England Journal of Medicine, 378(23), 2191-2201. https://doi.org/10.1056/NEJMoa1802686
