LEADER
What is the effect of liraglutide in patients with T2DM and high cardiovascular risk?
Study design
Population
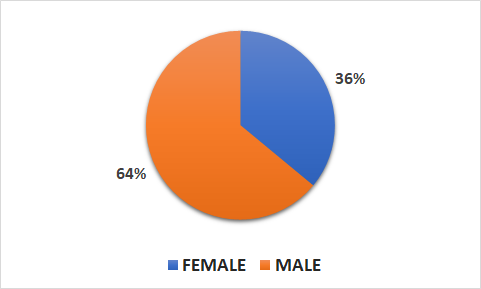
- 9340 patients (3337 female, 6003 male)
- Inclusion criteria: patients with T2DM who had a glycated Hgb level of ≥ 7.0% and high cardiovascular risk
- Key exclusion criteria: T1DM, calcitonin ≥ 50 ng/L, chronic HF, current continuous RT, end-stage renal disease, history of solid organ transplant or awaiting solid organ transplant, malignant neoplasm, personal history of non-familial medullary thyroid carcinoma
Interventions
- N=4668 liraglutide (1.8 mg or maximum tolerated dose once daily as a subcutaneous injection in addition to standard care)
- N=4672 placebo (matching placebo once daily as a subcutaneous injection in addition to standard care)
Primary outcome
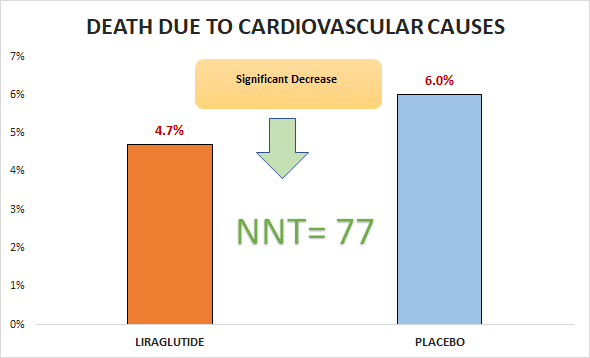
Significant decrease in death due to cardiovascular causes (4.7% vs. 6%; HR 0.78, 95% CI 0.66 to 0.93)
Safety outcomes
- No significant differences in adverse events (62.3% VS.
60.8%, p=0.12) and serious adverse events (49.7% VS.
50.4%, p=0.51). - Significant differences in adverse event leading to discontinuation of trial regimen (9.5% vs. 7.3%, p <0.001) and acute gallstone disease (3.1% Vs. 1.9%, p <0.001).
Conclusion
In patients with T2DM who had a glycated Hgb level of ≥ 7.0% and high cardiovascular risk, liraglutide was noninferior to placebo with respect to death due to cardiovascular causes.
