EMPA-REG
What is the role of empagliflozin in patients with T2DM at high cardiovascular risk?
Study design
Population
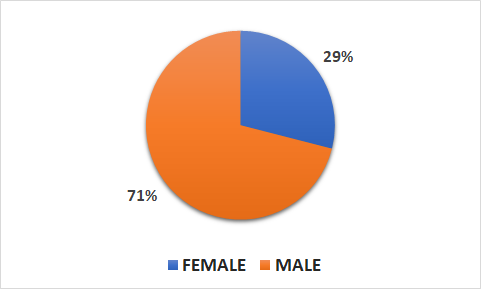
- 7020 patients (2004 female, 5016 male)
- Inclusion criteria: patients with T2DM at high cardiovascular risk who are on standard care
- Key exclusion criteria: uncontrolled hyperglycemia with glucose > 240 mg/dL, indication of liver disease, planned cardiac surgery or angioplasty within 3 months, bariatric surgery within the past 2 years, blood dyscrasias, medical history of cancer and/or treatment of cancer within the last 5 years, uncontrolled endocrine disorder, or pregnant or lactating women
Interventions
- N=4687 empagliflozin (10 mg or 25 mg once daily)
- N=2333 placebo (matching placebo once daily)
Primary outcome
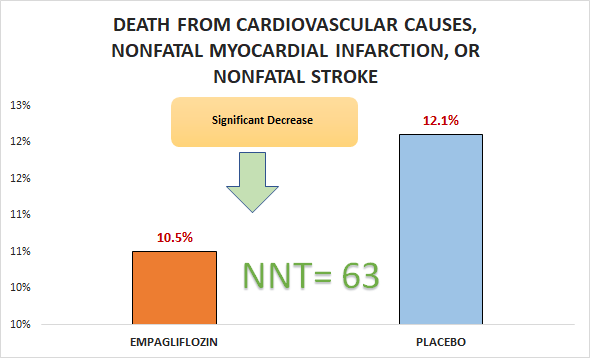
Significant decrease in death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (10.5% vs. 12.1%; HR 0.86, 95% CI 0.74 to 0.99)
Secondary outcomes
Significant decrease in cardiovascular death (3.7% VS.
5.9%; HR 0.62, 95% CI 0.49 to 0.77)
Safety outcomes
- No significant differences in adverse events, serious adverse events and adverse events leading to discontinuation of study drug.
- Significant difference in genital infection (6.4% VS.
1.8%).
Conclusion
In patients with T2DM at high cardiovascular risk who are on standard care, empagliflozin was superior to placebo with respect to death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke.
