What is the role of dapagliflozin among patients with HFrEF?
Study design
Population
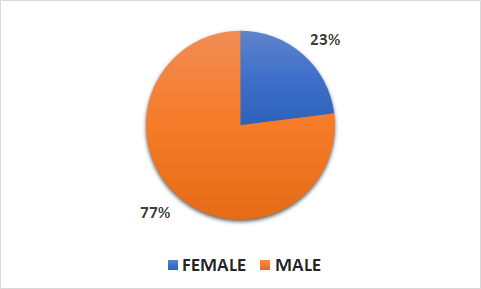
- 4744 patients (1109 female, 3635 male)
- Inclusion criteria: adult patients with NYHA class II, Ill, or IV HF and an ejection fraction < 40%
- Key exclusion criteria: recent treatment with or unacceptable side effects associated with a sodium-glucose cotransporter 2 inhibitor, T1DM mellitus, symptoms of hypotension or a systolic BP < 95 mmH
Interventions
- N=2373 dapagliflozin (at a dose of 10 mg once daily, per oral use)
- N=2371 placebo (matching placebo daily)
Primary outcome
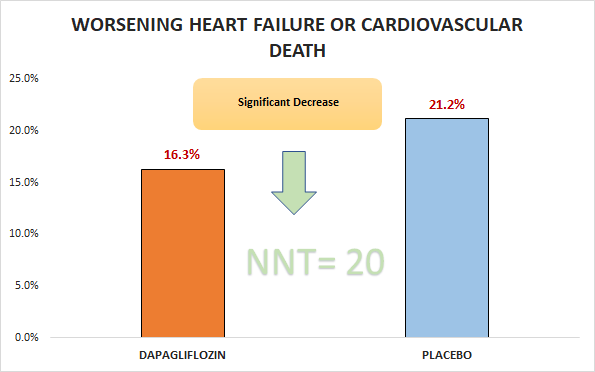
Significant decrease in worsening HF or cardiovascular death (16.3% Vs. 21.2%; HR 0.74, 95% CI 0.65 to 0.85)
Secondary outcomes
- Significant decrease in hospitalization for HF or death from cardiovascular causes (16.1% Vs. 20.9%; HR 0.75, 95% CI 0.65 to 0.85)
- Borderline significant decrease in a first worsening cardiovascular event (10% vs. 13.7%; HR 0.7, 95% CI 0.59 to 0.83)
- Borderline significant decrease in death from any cause (11.6% Vs. 13.9%; HR 0.83, 95% CI 0.71 to 0.97)
Safety outcomes
- No significant differences in adverse events including volume depletion, hypoglycemia, renal dysfunction.
- Significant difference in serious renal adverse event (1.6% VS. 2.7%).
Conclusion
In adult patients with NYHA class lI, Ill, or IV HF and an ejection fraction < 40%, dapagliflozin was superior to placebo with respect to worsening HF or cardiovascular death.
