COPERNICUS
What is the role of carvedilol on the morbidity of patients with severe chronic HF?
Study design
Population
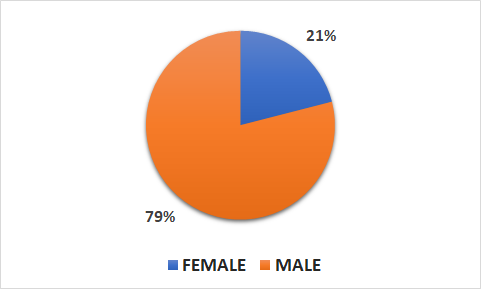
- 2289 patients (470 female, 1819 male)
- Inclusion criteria: patients with symptoms of HF at rest or on minimal exertion and with an ejection fraction <
25% (but not volume-overloaded) - Key exclusion criteria: HF caused by uncorrected primary valvular disease or a reversible form of cardiomyopathy; severe primary pulmonary, renal, or hepatic disease; or contraindication to B-blocker therapy
Interventions
- N=1156 carvedilol (target dose of 25 mg BID for an average of 10.4 months)
- N=1133 placebo (matching placebo for an average of 10.4 months)
Primary outcome
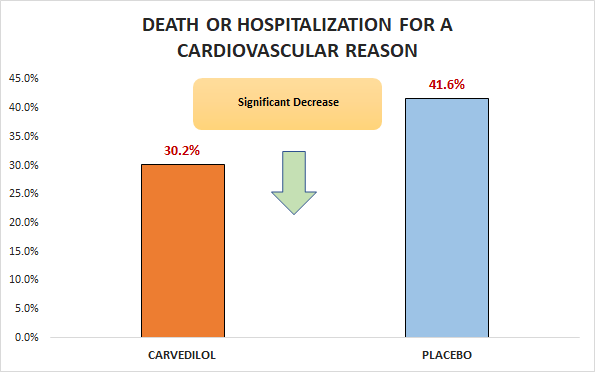
Significant decrease in death or hospitalization for a cardiovascular reason (30.2% vs. 41.6%; RR 0.73, 95% CI 0.63 to 0.84)
Secondary outcomes
Significant decrease in death or hospitalization for HF (25.5% vs. 37.9%; RR 0.69, 95% CI 0.59 to 0.81)
Safety outcomes
Significant differences in serious adverse event (39.0% vs. 45.5%, p = 0.002).
Conclusion
In patients with symptoms of HF at rest or on minimal exertion and with an ejection fraction < 25% (but not volume-overloaded), carvedilol was superior to placebo with respect to death or hospitalization for a cardiovascular reason.
