Baclofen for Alcoholism
To evaluate the benefits and harms of baclofen on achieving and maintaining abstinence or reducing alcohol consumption in people with AUD compared to placebo, no treatment, or any other pharmacological relapse prevention treatment.
Study design
Systematic review and meta-analysis of 17 randomized controlled trials
Population
- Number of patients studied: 1818 participants
- Inclusion criteria: Participants with a diagnosis of alcohol dependence according to the DSM or International Classification of Diseases 10th edition criteria.
- Exclusion criteria: Studies with treatment duration of less than four weeks or overall study duration of less than 12 weeks.
Interventions
- Experimental group: Baclofen (daily doses ranging from 30 mg to 300 mg)
- Control group: Placebo, acamprosate, or naltrexone
Primary outcome
- Risk of relapse, percentage of abstinent days, heavy drinking days, number of drinks per drinking days, and adverse events.
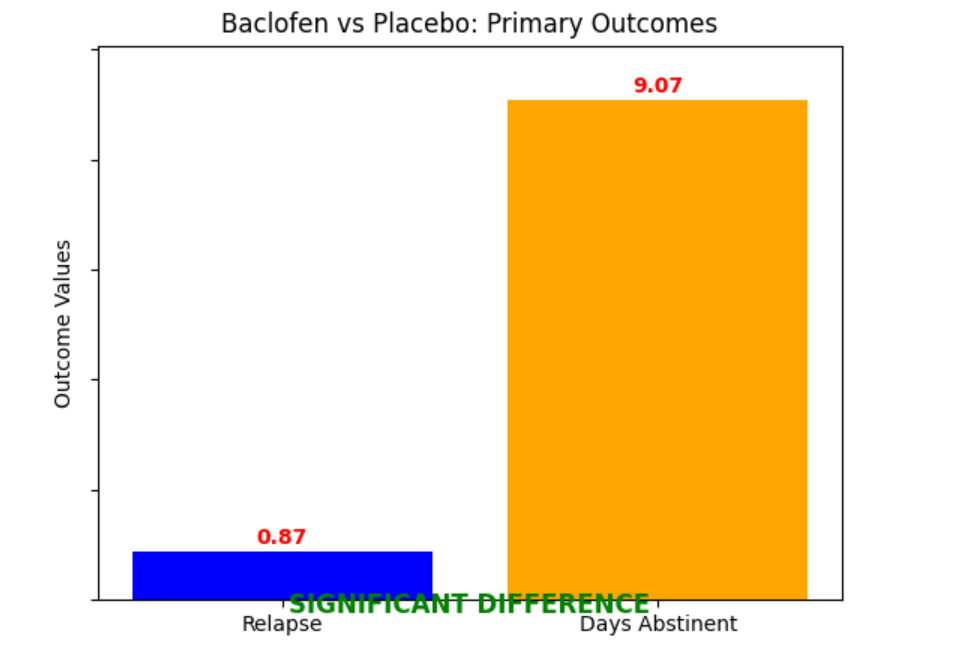
Significant difference: Yes, baclofen likely reduces the risk of relapse to any drinking (RR 0.87, 95% CI 0.77 to 0.99) and increases the percentage of abstinent days (MD 9.07, 95% CI 3.30 to 14.85).
Secondary outcomes
No significant differences in craving, anxiety, and depression between baclofen and placebo groups.
Safety outcomes or Adverse Events
Baclofen did not increase the number of participants with at least one adverse event or those who dropped out for any reason or due to adverse events. Baclofen increased fatigue, dizziness, somnolence/sedation, dry mouth, paraesthesia, and muscle spasms/rigidity.
Conclusion
Baclofen likely reduces the risk of relapse to any drinking and increases the percentage of abstinent days, mainly among detoxified participants
