AID-ICU
In adult patients admitted to the ICU that develop hyperactive or hypoactive delirium, does scheduled and as needed intravenous haloperidol as compared to placebo affect the number of days alive and out of the hospital?
Study design
Multicenter, double-blind, randomized, placebo-controlled trial
Population
- Number of patients studied: 1000
- Inclusion criteria: ≥18 years old with unplanned admission to an ICU for an acute condition, screened positive for delirium with CAM-ICU or ICDSC
- Exclusion criteria: Haloperidol contraindication, non-pharmacologic coma, regular anti-psychotic therapy, permanently incompetent patient, withdrawal from active therapy, brain death, pregnancy, involuntary hospitalization, alcohol withdrawal
Interventions
- Experimental group: Haloperidol at 2.5mg IV three times daily, plus as needed doses of 2.5mg up to a daily maximum of 20mg
- Control group: Matched placebo of both scheduled and as needed doses
Primary outcome
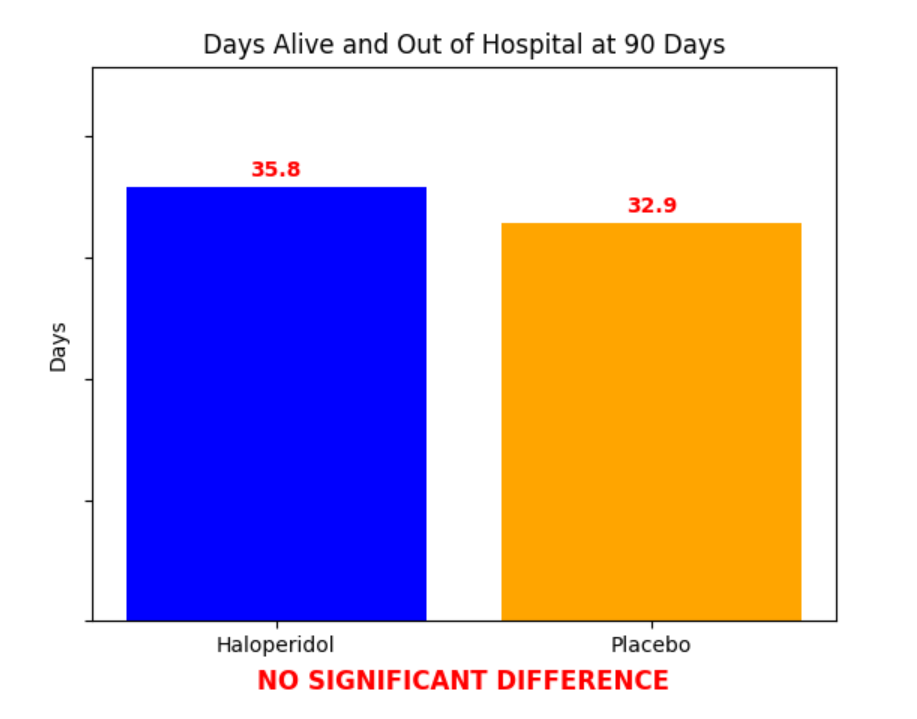
Significant difference? No (P = 0.22)
Secondary outcomes
All-cause mortality at 90 days: 36.3% in the haloperidol group vs. 43.3% in the placebo group (adjusted RR 0.84; 95% CI 0.72 to 0.98)
Safety outcomes or Adverse Events
Serious adverse reactions in ICU: 2.2% in the haloperidol group vs. 1.9% in the placebo group
Therapy stopped due to QTc prolongation: 2.4% in the haloperidol group vs. 1.4% in the placebo group
Physical restraints utilized: 1.9% in the haloperidol group vs. 2.1% in the placebo group
Andersen-Ranberg NC, et al. "Haloperidol for the Treatment of Delirium in ICU Patients". The New England Journal of Medicine. 2022. Online ahead of print(2022-10-26):1-11.
