ACCOMPLISH
Is benazepril plus amlodipine superior to benazepril plus hydrochlorothiazide in patients with HTN who are at high-risk for cardiovascular events?
Study design
Population
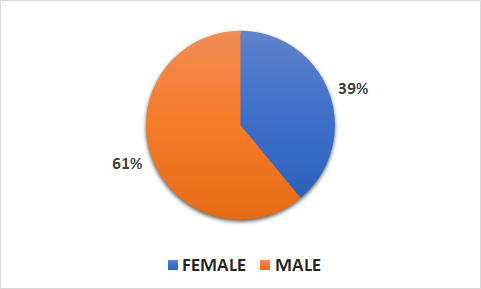
- 11506 patients (4542 female, 6963 male)
- Inclusion criteria: patients with HTN who were at high risk for cardiovascular events
- Key exclusion criteria: ACSs, or coronary revascularizations within 1 month of the first visit; stroke or other ischemic cerebrovascular episodes within 3 months preceding study evaluation; or HTN that is excessively severe, known to be refractory to treatment, or known to have a secondary cause
Interventions
- N=5744 benazepril-amlodipine (20 mg benazepril and 5 mg amlodipine)
- N=5762 benazepril-hydrochlorothiazide (20 mg benazepril and 12.5 mg hydrochlorothiazide)
Primary outcome
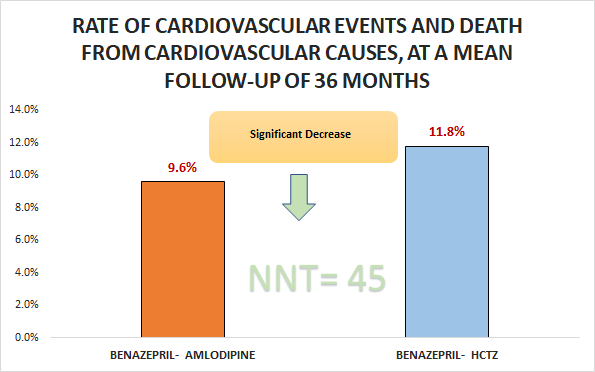
Significant decrease in the rate of cardiovascular events and death from cardiovascular causes, at a mean follow-up of 36 months (9.6% vs. 11.8%; HR 0.8, 95% CI 0.72 to 0.9)
Secondary outcomes
- Significant decrease in death from cardiovascular causes, nonfatal myocardial infarction, and nonfatal stroke (5% vs. 6.3%; HR 0.79, 95% Cl 0.67 to 0.92)
- Significant decrease in cardiovascular events (8.6% vs. 10.3%; HR 0.83, 95% CI 0.73 to 0.93)
Safety outcomes
- No significant difference in dry cough (20.5% vs. 21.2%) and hyperkalemia (0.6% vs. 0.6%).
- Significant differences in peripheral edema (31.2% vs.13.4%), dizziness (20.7% vs. 25.4%), and hypotension (2.5% vs. 3.6%).
Conclusion
In patients with HTN who were at high risk for cardiovascular events, benazepril-amlodipine was superior to benazepril-hydrochlorothiazide with respect to the rate of cardiovascular events and death from cardiovascular causes, at a mean follow-up of 36 months.
